Periodic Table of Elements (Downloadable) and How to Read It
Home >> Excel Tutorials from Compute Expert >> Excel Tips and Trick >> Periodic Table of Elements (Downloadable) and How to Read It
In this article, you can see and download a periodic table of elements in excel and/or PDF files. You can also learn how to read and optimally use the table and how to create the table in excel yourself.
Excel is very flexible software and we can program it into many things. One useful thing we can make from it is the periodic table of elements. As long as you master all the functions you need, you can create a neat, customized periodic table for yourself quickly.
Want to learn from the periodic table, download it, and/or understand how to read it in the right way? Stay tuned!
Disclaimer: This post may contain affiliate links from which we earn commission from qualifying purchases/actions at no additional cost for you. Learn more
Want to work faster and easier in Excel? Install and use Excel add-ins! Read this article to know the best Excel add-ins to use according to us!
Table of Contents:
- Our periodic table of elements content
- Periodic table of elements download (excel xlsx/PDF)
- How to read a periodic table of elements
- Elements grouping and classification in a periodic table of elements
- Tips to use/learn this periodic table of elements
- How to create the table in excel
- How to modify the table in its excel template
- Periodic table of elements exercise
- Additional note
Our Periodic Table of Elements Content
Here is a snapshot of the periodic table of elements we have created for you.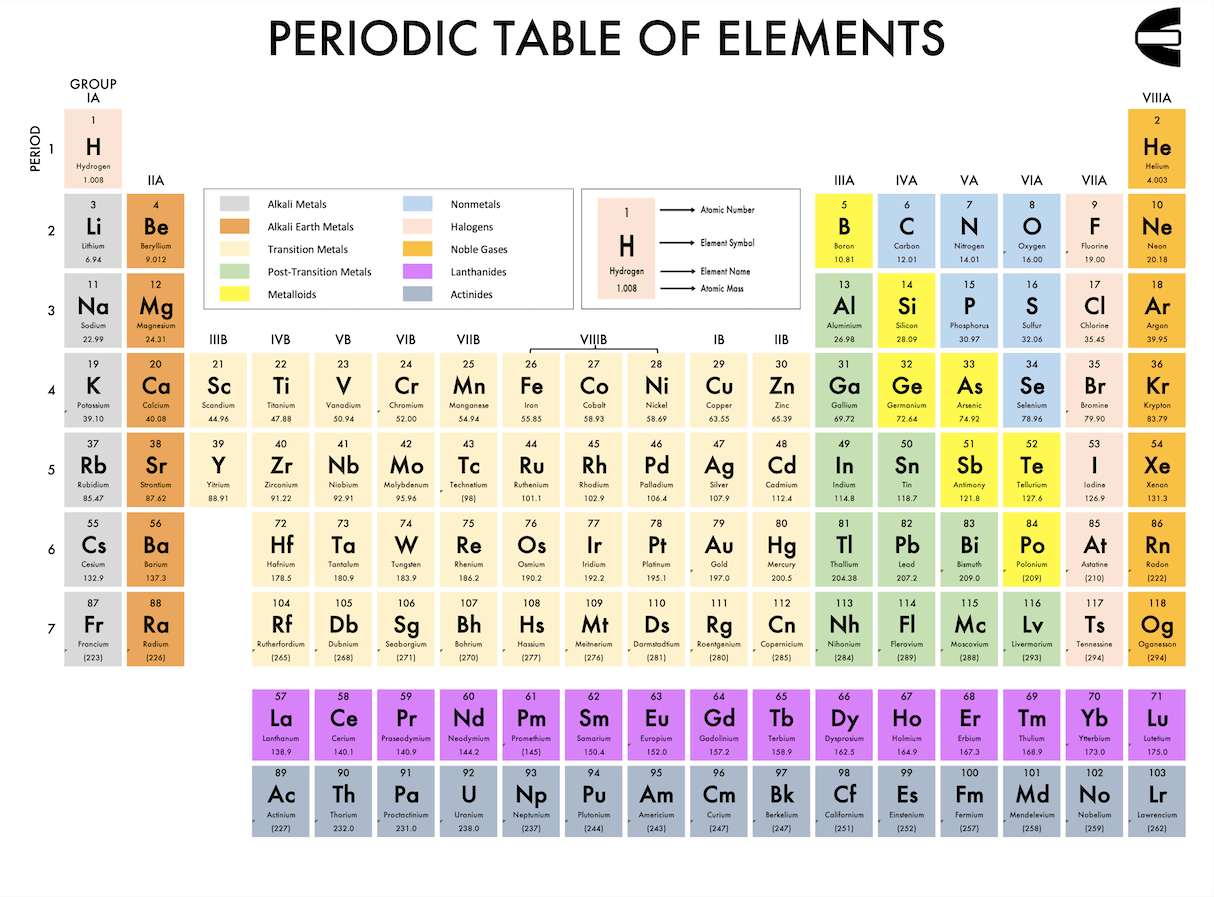
If you download the excel file of this periodic table of elements, you can edit the table display to your preference.
Below is the list of the chemical elements that have a place in the periodic table.
| Atomic Number | Element Symbol | Element Name | Classification |
|---|---|---|---|
| 1 | H | Hydrogen | Halogen |
| 2 | He | Helium | Noble Gas |
| 3 | Li | Lithium | Alkali Metal |
| 4 | Be | Beryllium | Alkali Earth Metal |
| 5 | B | Boron | Metalloid |
| 6 | C | Carbon | Nonmetal |
| 7 | N | Nitrogen | Nonmetal |
| 8 | O | Oxygen | Nonmetal |
| 9 | F | Fluorine | Halogen |
| 10 | Ne | Neon | Noble Gas |
| 11 | Na | Sodium | Alkali Metal |
| 12 | Mg | Magnesium | Alkali Earth Metal |
| 13 | Al | Aluminium | Post-Transition Metal |
| 14 | Si | Silicon | Metalloid |
| 15 | P | Phosphorus | Nonmetal |
| 16 | S | Sulfur | Nonmetal |
| 17 | Cl | Chlorine | Halogen |
| 18 | Ar | Argon | Noble Gas |
| 19 | K | Potassium | Alkali Metal |
| 20 | Ca | Calcium | Alkali Earth Metal |
| 21 | Sc | Scandium | Transition Metal |
| 22 | Ti | Titanium | Transition Metal |
| 23 | V | Vanadium | Transition Metal |
| 24 | Cr | Chromium | Transition Metal |
| 25 | Mn | Manganese | Transition Metal |
| 26 | Fe | Iron | Transition Metal |
| 27 | Co | Cobalt | Transition Metal |
| 28 | Ni | Nickel | Transition Metal |
| 29 | Cu | Copper | Transition Metal |
| 30 | Zn | Zinc | Transition Metal |
| 31 | Ga | Gallium | Post-Transition Metal |
| 32 | Ge | Germanium | Metalloid |
| 33 | As | Arsenic | Metalloid |
| 34 | Se | Selenium | Nonmetal |
| 35 | Br | Bromine | Halogen |
| 36 | Kr | Krypton | Noble Gas |
| 37 | Rb | Rubidium | Alkali Metal |
| 38 | Sr | Strontium | Alkali Earth Metal |
| 39 | Y | Yitrium | Transition Metal |
| 40 | Zr | Zirconium | Transition Metal |
| 41 | Nb | Niobium | Transition Metal |
| 42 | Mo | Molybdenum | Transition Metal |
| 43 | Tc | Technetium | Transition Metal |
| 44 | Ru | Rutherium | Transition Metal |
| 45 | Rh | Rhodium | Transition Metal |
| 46 | Pd | Palladium | Transition Metal |
| 47 | Ag | Silver | Transition Metal |
| 48 | Cd | Cadmium | Transition Metal |
| 49 | In | Indium | Post-Transition Metal |
| 50 | Sn | Tin | Post-Transition Metal |
| 51 | Sb | Antimony | Metalloid |
| 52 | Te | Tellurium | Metalloid |
| 53 | I | Iodine | Halogen |
| 54 | Xe | Xenon | Noble Gas |
| 55 | Cs | Cesium | Alkali Metal |
| 56 | Ba | Barium | Alkali Earth Metal |
| 57 | La | Lanthanum | Lanthanide |
| 58 | Ce | Cerium | Lanthanide |
| 59 | Pr | Praseodymium | Lanthanide |
| 60 | Nd | Neodymium | Lanthanide |
| 61 | Pm | Promethium | Lanthanide |
| 62 | Sm | Samarium | Lanthanide |
| 63 | Eu | Europium | Lanthanide |
| 64 | Gd | Gadolinium | Lanthanide |
| 65 | Tb | Terbium | Lanthanide |
| 66 | Dy | Dysprosium | Lanthanide |
| 67 | Ho | Holmium | Lanthanide |
| 68 | Er | Erbium | Lanthanide |
| 69 | Tm | Thulium | Lanthanide |
| 70 | Yb | Ytterbium | Lanthanide |
| 71 | Lu | Lutetium | Lanthanide |
| 72 | Hf | Hafnium | Transition Metal |
| 73 | Ta | Tantalum | Transition Metal |
| 74 | W | Tungsten | Transition Metal |
| 75 | Re | Rhenium | Transition Metal |
| 76 | Os | Osmium | Transition Metal |
| 77 | Ir | Iridium | Transition Metal |
| 78 | Pt | Platinum | Transition Metal |
| 79 | Au | Gold | Transition Metal |
| 80 | Hg | Mercury | Transition Metal |
| 81 | Tl | Thallium | Post-Transition Metal |
| 82 | Pb | Lead | Post-Transition Metal |
| 83 | Bi | Bismuth | Post-Transition Metal |
| 84 | Po | Palonium | Metalloid |
| 85 | At | Astatine | Halogen |
| 86 | Rn | Radon | Noble Gas |
| 87 | Fr | Francium | Alkali Metal |
| 88 | Ra | Radium | Alkali Earth Metal |
| 89 | Ac | Actinium | Actinide |
| 90 | Th | Thorium | Actinide |
| 91 | Pa | Proctactinium | Actinide |
| 92 | U | Uranium | Actinide |
| 93 | Np | Neptunium | Actinide |
| 94 | Pu | Plutonium | Actinide |
| 95 | Am | Americium | Actinide |
| 96 | Cm | Curium | Actinide |
| 97 | Bk | Berkelium | Actinide |
| 98 | Cf | Californium | Actinide |
| 99 | Es | Einstenium | Actinide |
| 100 | Fm | Fermium | Actinide |
| 101 | Md | Mendelevium | Actinide |
| 102 | No | Nobelium | Actinide |
| 103 | Lr | Lawrencium | Actinide |
| 104 | Rf | Rutherfordium | Actinide |
| 105 | Db | Dubnium | Actinide |
| 106 | Sg | Seaborgium | Actinide |
| 107 | Bh | Bahrium | Actinide |
| 108 | Hs | Hassium | Actinide |
| 109 | Mt | Meitnerium | Actinide |
| 110 | Ds | Darmstadtium | Actinide |
| 111 | Rg | Roentgenium | Actinide |
| 112 | Cn | Copernicium | Actinide |
| 113 | Nh | Nihonium | Post-Transition Metal |
| 114 | Fl | Flerovium | Post-Transition Metal |
| 115 | Mc | Moscovium | Post-Transition Metal |
| 116 | Lv | Livermorium | Post-Transition Metal |
| 117 | Ts | Tennessine | Halogen |
| 118 | Og | Oganesson | Noble Gas |
Periodic Table of Elements Download (Excel xlsx/PDF)
You can find the link to download the excel and PDF version of the table below.If you want to edit the display of our periodic table to your preference, you should download the excel file. After you edit it in the excel file, you can export it to PDF or print it directly if you want.
We provide the table PDF in A3 and A4 versions here. If you intend to print the table to one of those papers, then download the PDF version you need.
Excel xlsx
PDF (A3)
PDF (A4)
How to Read a Periodic Table of Elements
A periodic table of elements places and groups the elements which existence we have identified based on their nature. You may notice some gaps that the periodic table of elements has in the middle. That is because we place the transition metals group there which nature is quite different from other elements.The atomic number for each element (the number at the top of each element symbol in the table) represents the number of protons that element has. For example, as Nickel’s atomic number is 28 and Cobalt’s is 27, that means Nickel has more protons than Cobalt.
In the table, the elements are divided into periods (rows) and groups (columns). The elements that are in the same period have the same number of orbitals. The number of orbitals ranges from 1 (which the elements in the top period have) to 7 (which elements in the bottom period have).
The elements in the same group, on the other hand, have the same number of electrons in their outer shell. They also have similar physical properties and similar reactions to the elements in another group.
The relative position of one element to another in the periodic table of elements also has meanings. The more right an element location is in the periodic table, the larger ionization energy the element has.
It will also be more nonmetal in nature and has more negative electron affinity. Meanwhile, the more above an element is in the periodic table, the larger its electronegativity is.
Furthermore, the more right and above an element position is, the larger the atomic radius the element has. That is because the larger the element’s atomic number is, the more electrons it has. That means it will have a larger electron trajectory and therefore, a larger atomic radius.
Elements Grouping and Classification in a Periodic Table of Elements
As you can see in one of our periodic table of elements legend, the table divides the elements into ten groups. They are the alkali metals, alkali earth metals, transition metals, post-transition metals, metalloids, nonmetals, halogens, noble gases, lanthanides, and actinides.The members of these ten groups and their general characteristic in brief are:
- Alkali metals (Li, Na, K, Rb, Cs, Fr): extremely reactive and will burst into flame when contacting water
- Alkali earth metals (Be, Mg, Ca, Sr, Ba, Ra): reactive but not as reactive as alkali metals
- Transition metals (Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Rf, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn): malleable, shiny, and are a good conductor
- Lanthanides (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu): have a silver color and tarnish when contacting air
- Actinides (Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr): radioactive
- Post-transition metals (Al, Ga, In, Sn, Tl, Pb, Bi, Nh, Fl, Mc, Lv): have characteristics similar to transition metals but softer and are a worse conductor
- Metalloids (B, Si, Ge, As, Sb, Te, Po): have conductor/semiconductor properties
- Nonmetals (C, N, O, P, S, Se): are a poor conductor
- Halogens (F, Cl, Br, I, At, Ts): quite reactive chemically
- Noble gases (He, Ne, Ar, Kr, Xe, Rn, Og): colorless, odorless, and nonreactive
We can group these ten again into three big groups if we want. These three big groups are metals, metalloids, and nonmetals.
Each of these three big groups has different traits generally. Those traits for each big group are:
- Metals (alkali metals, alkali earth metals, lanthanides, actinides, transition metals, and post-transition metals):
- A good heat and electricity conductor
- Malleable and ductile
- Solid at room temperature
- Metalloids (metalloids):
- Have physical properties more like nonmetals
- Under certain circumstances, several metalloids can conduct electricity
-
Nonmetals (nonmetals, halogens, and noble gases):
- A poor heat and electricity conductor
- Not malleable and not ductile
- Many are gases at room temperature
You can easily see the elements groupings according to the ten groups and three big groups in their periodic table placement.
Tips to Use/Learn This Periodic Table of Elements
Need to learn the details of the elements in the periodic table? Here are some tips you can apply to get the most of our periodic table of elements.- Print the PDF A4 version of our periodic table and put it somewhere you can easily take. Moreover, print the PDF A3 version too and place it in a strategic place you can easily see. That way, you will grow more accustomed to the table content and able to learn it wherever whenever you want
- Make rhymes, songs, stands, or sentences for the elements sequence in each period and group in the table. By doing that, you should memorize the element members of each period and group much easier
- Break the table into sections and repeat the memorization of each section separately. Do it until you can recite them easily before you group the sections again into one
Hope these tips can help you learn more easily!
How to Create the Table in Excel
Want to learn how to create a periodic table of elements in excel? It is very simple and you should be able to do it if you have mastered the basic functions in excel!You just need to understand how to insert and resize font, shapes, rows, and columns in excel. You should also be able to copy, color, and merge cells. As you may infer from our periodic table display, we created the table partly by doing these things.
For the periodic table legends, we created them in the second sheet. We didn’t create them directly in the first sheet since the cells form there seem to won’t display the legends neatly. Therefore, we copy the legends we created in the second sheet and paste them as pictures in the first sheet.
For the table background, we color it white since we want it not to be transparent. You may also notice that we inserted rows and columns in between the elements there. This is so we can separate those elements location clearly.
How to Modify this Table in Its Excel Template
Want to modify the display of our periodic table of elements in its excel file? You should be able to do most things we did to create the table beforehand!You may want to change the color, font, or cell details of the table. You can do that easily by mastering the ways to alter the worksheet display in excel.
If you need to change the display/content of the table legends, you can do that in the second sheet. Do the modifications you want and then copy-paste them as pictures in the first sheet after you finish.
Multiplication Exercise/Game
Have learned from the periodic table of elements and the contents of this article? Want to test your knowledge by doing some related exercise? Try to answer the five questions below!- What are the metalloid and noble gas elements?
- What is the group of hydrogen, magnesium, and gold in the periodic table of elements?
- What is the meaning of the left-to-right position of the elements in the periodic table?
- What are the differences between metals, metalloids, and nonmetals and what are their element groups?
- What is the meaning of the atomic number that each element has in the periodic table?
If you don’t know the answers, check your periodic table and learn from this article content again!
Additional Note
To retrieve the information about an element from our periodic table in excel, you can use the HLOOKUP or INDEX MATCH. You may want to create a separate table first though that contains all the elements information you need. This is so you can retrieve the information you need later much easier.Excel tutorials you may want to learn: